You may be aware that patients' expectations for regenerative medicine have been increasing more and more.
However, you may also understand that patients with intractable diseases cannot be treated with regenerative medicine products immediately.
In this column, we will explain what conditions are required to apply regenerative medicine to actual treatment and the specified medical care coverage system for clinical trials.
Conditions for use of regenerative medicine products
In our previous column, we explained that the definition of "regenerative medicine products" in Japan does not refer to products used for regenerative medicine but to "gene and cell therapy products.
> Regenerative Medicine Column: What is “Regenerative medicine”?(This will open in a new window.)
Among such regenerative medicine products, some of them have been approved as regenerative medicine products for diseases that cannot be expected to be cured naturally due to injuries, etc. These products have been used in actual medical settings.
(Example) Spinal cord injury, Corneal epithelial stem cell deficiency, Severe heart failure and Articular cartilage deficiency
However, in some cases, even if you ask your doctor to treat you with a regenerative medicine product, it might not be possible.
Since it is stated that "participation in training sessions" and "use of this product after receiving training" are required in the package inserts of regenerative medicine products, medical treatment using such regenerative medicine products can be received only at limited facilities with a certain level of systems in Japan.
Necessity of prior training is clearly stated in the GCP for regenerative medicine products from the clinical trial stage.

Investigators who use regenerative medicine products can reduce variations in results and failures due to the style of the procedure and familiarity/unfamiliarity of the investigators with the handling of the trial products by conducting training in advance with animal models and practice kits under the presence of the sponsor, thereby improving treatment outcomes.
The training also allows the investigators to perform transplantation procedures with confidence.
Furthermore, since regenerative medicine products are produced from living cells and viruses, not only to investigators who actually use the products but also to those in charge of managing the products at the medical facilities or preparing the products before the administration need to take the training. For this reason, prior training is very important for regenerative medicine products which require procedures when the products are used.
Even after obtaining marketing approval, treatment outcome research is required for regenerative medicine products as all cases must be investigated in principle, and training is also required at the medical institutions where the products are adopted.
![[Reference] Necessity of training described in the package inserts for regenerative medicine products](/assets/images/en/column/rgm/column_202102ins_img04.png)
It may not be easy for some facilities to set up this kind of training system.
Since Remedy has wide experience in the development of regenerative medicine products, we can offer clinical trial product training as well as services for product development and post-marketing trials.
If you have any questions about the field of regenerative medicine products after reading this column, please contact us via <Inquiry Form>(This will open in a new window.).
About specified medical care coverage system for clinical trials of regenerative medicine products
Once the system is in place and it becomes possible to conduct clinical trials of regenerative medicine products at medical facilities, the next concern would be about the cost burden.
In Japan, the universal health insurance system allows people to receive medical treatment at low cost. This system is limited to medical services that are approved to be covered by insurance. Therefore, patients should pay full amount for medical treatment that are not covered by insurance.
In addition, in the case of mixed medical treatment, which is a combination of insured and uninsured medical treatment, patients are responsible for paying for medical treatment that is allowed by insurance as well.
Even if it is not a clinical trial for a regenerative medicine product, clinical trials commissioned by CROs fall under the category of mixed medical treatment because investigational drugs are not covered by insurance. However, since a clinical trial is defined as evaluation treatment (healthcare services to be evaluated), the specified medical care coverage system is applied for clinical trials.
Evaluation treatment refers to medical treatment that requires evaluation from the perspective of efficiently providing appropriate medical care to determine whether it should be applicable for insurance benefits.
The following are defined as "evaluation medical treatment"*1.

Everyone who participates in clinical trials may have struggled with the specified medical care coverage system.
As explained above, when insured and uninsured medical treatment are mixed, patients are responsible for paying the full amount, but this system allows insurance coverage for some medical treatment and pays for it as covered expenses.
Since the sponsor (pharmaceutical company, etc.) covers expenses for examinations that are not covered by insurance, subjects can participate in clinical trials with only the costs covered by insurance.
【Application of the specified medical care coverage system for regenerative medicine products】
So, can this system be applied throughout the clinical trial period?
The applicable period of the specified medical care coverage system for pharmaceutical products is from the start date of the investigational drug administration to the end date of the administration.
Unlike pharmaceutical products, regenerative medicine products are not often administered to patients on a continuous basis.
In addition, the post-observation period tends to be longer for drugs that stay in the subject's body for a long period of time because it takes time to evaluate the safety.
The applicable period and scope of the specified medical care coverage system for regenerative medicine products are summarized below in terms of differences from clinical trials for pharmaceutical products and medical devices described in the Health Insurance Announcement No. 1159*2. *3
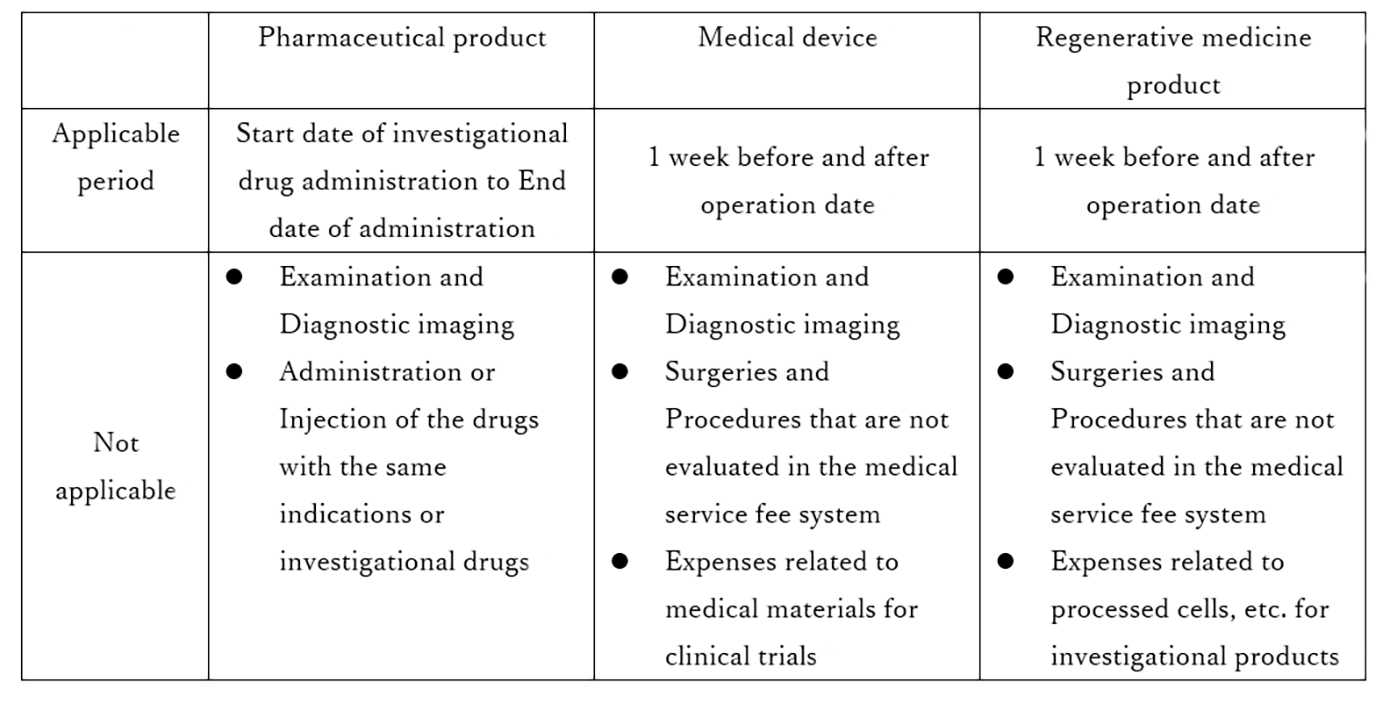
In clinical trials of regenerative medicine products where the post-observation period is longer, the post-observation period is usually covered by insurance, and therefore subjects must pay for their own expenses under the insurance system.
However, there are many medical institutions that require the sponsor to cover costs in order to reduce the burden on the subjects, since insurance is applied to examinations during the post-observation period (although the sponsor is not required to bear the costs under the system).
Therefore, intellim negotiates with medical institutions to ensure that the costs of examinations, etc. are appropriately shared by the sponsor and subjects when conducting clinical trials.
【Citations and References】
1: Outline of advanced medical care, Ministry of Health, Labour and Welfare https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryouhoken/sensiniryo/index.html(This will open in a new window.) (See Jan. 14, 2021)
2: Partial amendment of "Notes on the implementation of 'posted matters, etc., specified by the Minister of Health, Labour and Welfare based on the Rules for Medical Treatment, the Rules for Pharmaceutical Treatment, and the Standards for Medical Treatment' and 'drugs, etc., specified by the Minister of Health, Labour and Welfare relating to the specified medical care coverage system'", November 25, 2014
https://kouseikyoku.mhlw.go.jp/kinki/gyomu/bu_ka/shido_kansa/kikan_tsuchi/documents/261125-hoi9.pdf(This will open in a new window.) (See Jan. 14, 2021)
3: Regarding the manufacture of specific medical care expenses related to pharmaceuticals, Central Social Medical Insurance Councilhttps://www.mhlw.go.jp/shingi/2005/02/dl/s0209-6a2.pdf(This will open in a new window.) (See Jan. 14, 2021)