Service
Regenerative Medicine
Our Regenerative Medicine team was formed in December, 2015. With consultation services for both business and developmental planning, and an operational division for implementation, the Regenerative Medicine team can provide guidance in developing an optimal solution.
Remedy Group's Features of Regenerative Medicine
-
Highly Specialized Team with Extensive Experience
We are now capable of overseeing all areas available for development
Regenerative Medicine Specified by Government Order
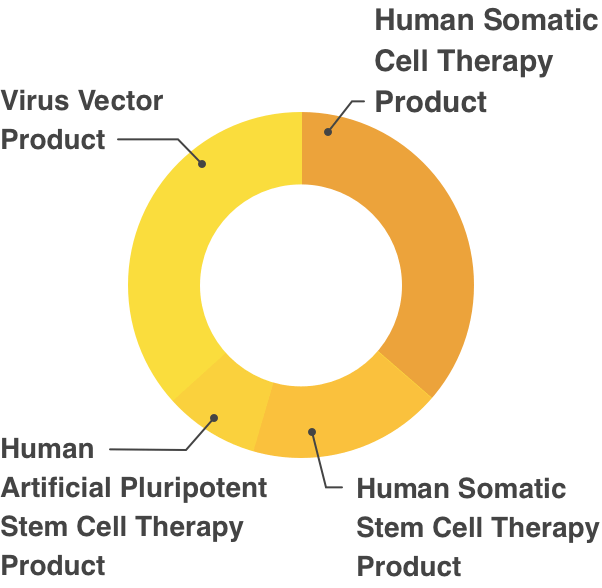
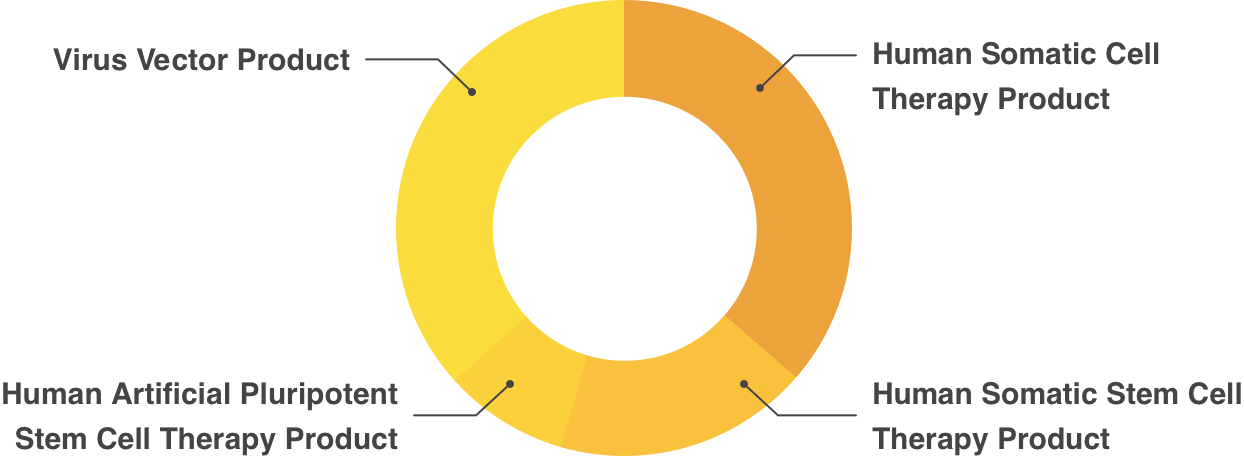
Partial Revision of Order for Enforcement of Pharmaceutical Affairs Law, appended Table 1 (Re Article 2)Human Cell/Tissue Products
- Human Somatic Cell Therapy Product
- Human Somatic Stem Cell Therapy Product
- Human Embryonic Stem Cell Therapy Product
- Human Artificial Pluripotent Stem Cell Therapy Product
Animal Cell/Tissue Products
- Animal Somatic Cell Therapy Product
- Animal Somatic Stem Cell Therapy Product
- Animal Embryonic Stem Cell Therapy Product
- Animal Artificial Pluripotent Stem Cell Therapy Product
Gene Therapy Products
- Plasmid Vector Product
- Virus Vector Product
- Gene Expression Therapy Product(Excluding those listed in preceding 2 items)
Performance Profile
-
Support to Rapidly Changing Regulations
The major difficulties of developing Regenerative Medicines are that regulations and evaluation methods have not yet caught up to the advancements in the technologies.
Remedy, is able to provide support to academia, healthcare institutions, and industry, through experience and accumulated data.Support Achievements
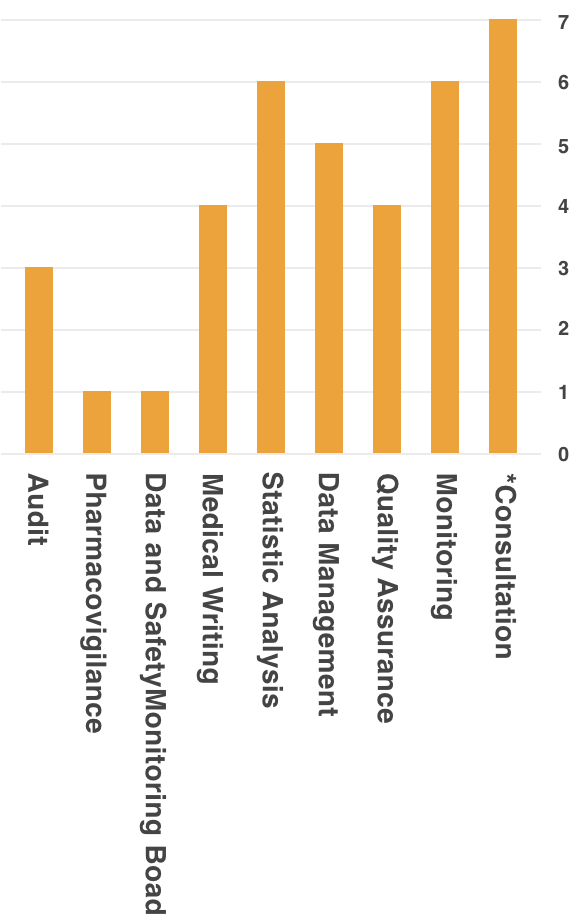
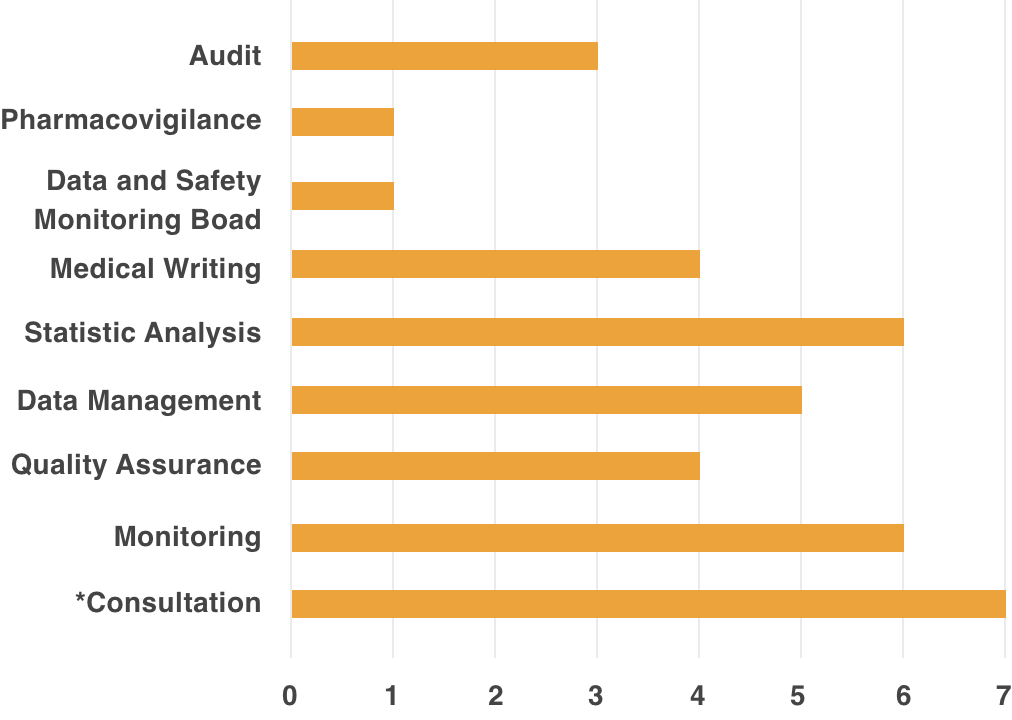
*Contents Of Consultation
- Consultation on Clinical Evaluation of small case of a few cases
- Support for implementing system of Clinical Trial (Creation of SOP Document, etc.)
- Development Strategy Planning Support (Exit Strategy)
- Clinical Trial Planning Support
- Consultation Support for PMDA/Various Authorities
- Response Support for Cartagena Act
- Support for Implementing Clinical Trial Operation in response to Cartagena Act
-
Fore-Sighted Market Valuation of Investment Value
We have formed a specialized team in the Regenerative Medicine business, capable of determining present investment value (NET Present Value: NPV) of projects based on various data. With thorough market valuation, we are capable of developing an effective strategy that provides an accurate investment value for our clients.
Points
- As a member of the Forum for Innovative Regenerative Medicine (FIRM), we are actively contributing to the advancement to Regenerative Medicine products
- We are focused in developing specialists that will lead the industry, through the implementation of a training program
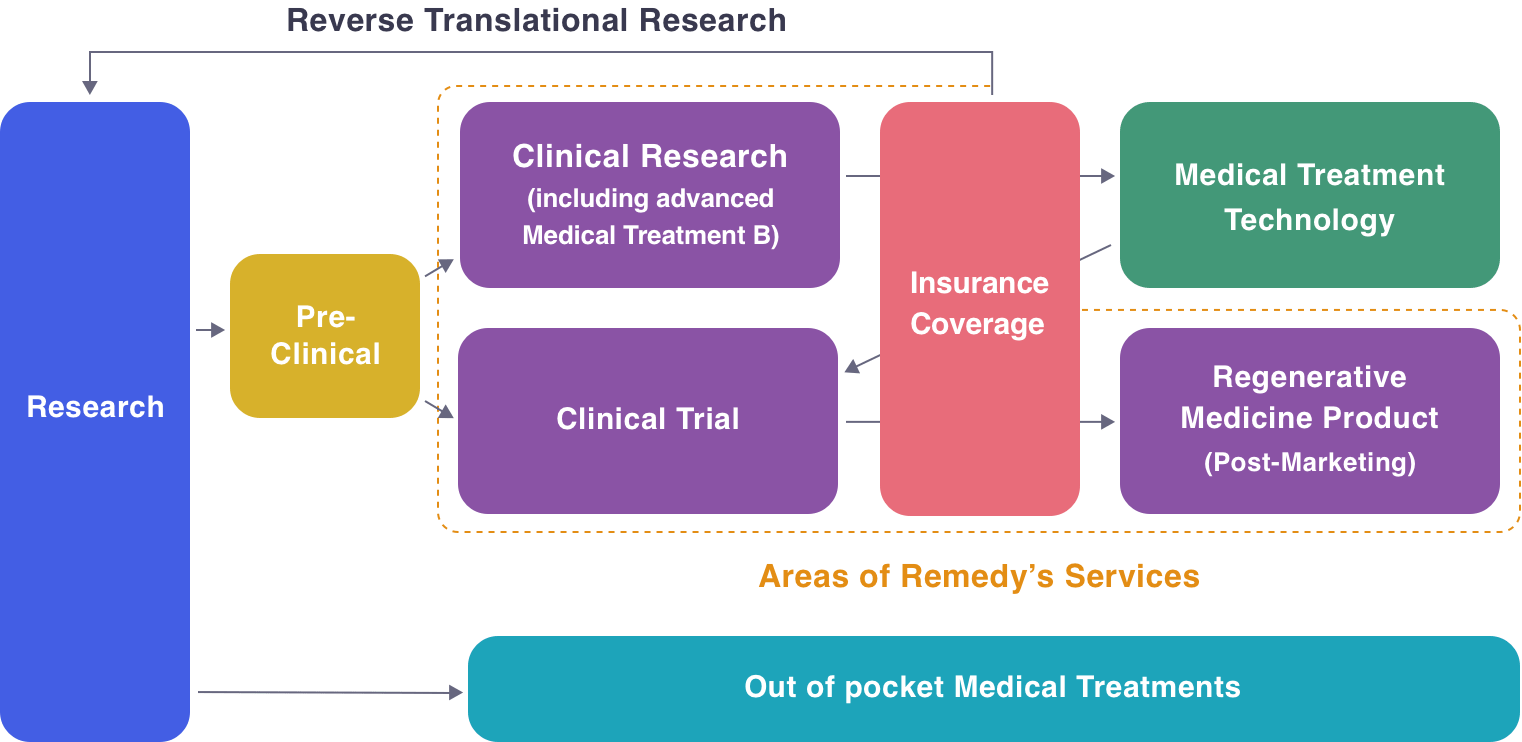
Starting From Strategy Development to Clinical Use
As Experts in the field of Regenerative Medicine,
We will propose the most optimal pathway to an Exit Strategy Starting From Strategy Development to Clinical Use