In the Pharmaceutical and Medical Device Act, “a regenerative medicine product” is defined as follows:
Article 2: The following (except quasi-pharmaceutical products and cosmetic items) are specified as “regenerative medicine products” in the Pharmaceutical and Medical Device Act:
- Products manufactured by culturing or processing cells of humans or animals for the purpose of the following medical or veterinary uses:
a) Formation of Structure or Mechanism of Human or Animal bodies
b) Treatment or Prevention of Diseases of Humans or Animals - Products which contain genes to be inserted in the cells of humans or animals and expressed in the bodies for the purpose of treatments
In the definition above, No. 1 indicates “a cell processed product”, and the No. 2 indicates “a gene therapy product”. Also, “cell processed products” are classified into the following 4 categories for the enforcement of the Pharmaceutical and Medical Device Act:
- Human cell-processed therapeutic products (except the following No. 2 and 4)
- Human somatic stem cell-processed therapeutic products (except the following No. 4)
- Human embryonic stem cell-processed therapeutic products
- Human induced pluripotent stem cell-processed therapeutic products
Cells used in treatments of diseases are classified into 3 types depending on its properties.
The first type is “pluripotential stem cells”. The cells are artificially produced, and can be transformed into all human cells forming our bodies (It can be said that there are approximately 270 types of cells including nerve cells, muscular cells and blood cells, etc.=These cells have the capability to differentiate into other cells). “Embryo-stem cells (ES cells)” and “induced pluripotent stem cells (iPS cells)" fall into this type.
The second type is “somatic stem cells”. The cells exist in our bodies and have the capability to differentiate into several different types of relevant cells. Hematopoietic stem cells (can differentiate into blood cells such as white blood cells, red blood cells and blood platelet, etc.”) and mesenchymal stem cells (can differentiate into osteoblast cells, adipose cells and myoblast cells, etc.) have been known as somatic stem cells.
The third type is “somatic cells”. Our bodies are formed with these somatic cells. All of these cells each have a unique function (insulin secretion of pancreatic islet beta-cellsm and red blood cells’ function of carrying oxygen, etc.), and the somatic cells don’t differentiate into other cells. In the Pharmaceutical and Medical Device Act, cell processed products are classified into 4 categories depending on the cell’s properties.
pluripotential stem cells such as ES cells and iPS cells are artificially produced, and don’t exist in our bodies. How are the pluripotential stem cells produced?
When you hear about cells having the capability to differentiate into any type of cell (about 270 types) as pluripotential stem cells, many of you may think of fertile ova. Fertile ovum has the capability to produce all cells forming our bodies with only one cell. Cells taken out from a fertile ovum and cultured become an ES cell (embryo(=featile avum)-stem cells).
ES cells inherit the capability to differentiate into all cells which fertile ova have. However, there are two issues in utilizing ES cells for treatments. The first is an ethical issue of destroying a fertile ovum which could grow in a human being if allowed to develop, for the sake of removing a cell.
The other is a technical issue, where the patient’s body may reject the transplanted cells, because ES cells are produced by cells from other people (It is impossible to produce ES cells from the patient’s own cells.). Due to these issues, clinical applications of ES cells had been have progressed at a sluggish pace.
The development of iPS cells changed this situation. Dr. Yamanaka proved that already differentiated cells can be restored to the initial state (the same state as fertile ova) in which cells can be differentiated into any type of cells again, by introducing four specific genes into the cells.
Dr. Yamanaka named these pluripotent stem cells artificially made by this initial phenomenon as iPS cells (induced Pluripotent Stem Cells). iPS cells can be produced from the patients’ own cells (skin cells, blood cells, etc.).
In iPS cell treatments, fertile ova are not destroyed, and there is no concern of the patient’s body rejecting the transplanted cells. Therefore, iPS cells solved at once both ES Cell issues – the need to destroy fertile ova and the risk of rejection.
As you know, since the advent of iPS cells, there have been attempts to use cells differentiated by iPS cells for the treatment of various diseases.
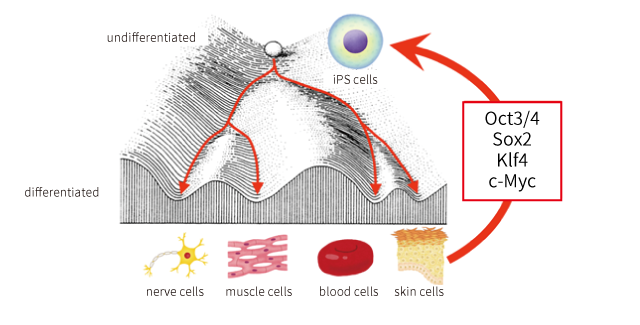
Pluripotent stem cells are not the only cells used to treat diseases. “somatic stem cells”, stem cells existing in our bodies, and differentiated “somatic cells” have been also used. mesenchymal stem cells are often cited as a somatic stem cell often used in disease treatments.
Among the regenerative medicine products approved in Japan, “TEMCELL® HS Inj” and “Stemilac injection” are both products using these mesenchymal stem cells.
On the other hand, products using already differentiated somatic stem cells, include “HeartSheet” using skeletal muscle myoblasts, “JACE®” using epidermal cells, “JACK” using condrocyte and “KYMRIAH®” using T cells, a form of lymphocytes.
Disclaimer: The information provided is not intended to provide any pharmaceutical or medical device regulatory law advice; instead, all information and content are for general informational purposes only.